Quartz Products
Pur Q® Fused Quartz Vials
Your breakthrough belongs in ours
Active pharmaceutical ingredients are becoming more complex, fragile and sensitive. At the same time, expectations for consistent product quality and purity are higher than ever.
Key Features
- Unrivaled chemical durability assures liquid stability and reliable long-term storage of even the most aggressive and sensitive formulations
- Ultra-high purity (>99.995% SiO2) eliminates risks associated with glass inhomogeneities (including delamination), as well as problematic metal ion leachables
- State-of-the-art tubing and vial manufacturing avoids glass-to-glass contact and delivers exceptional mechanical durability and cosmetic quality
- Industry-standard sizes (2ml–50ml) and tolerances guarantee no unwelcome manufacturing surprises as a drop-in material replacement
- As a proud FDA Emerging Technology Program participant Momentive Technologies has direct engagement with regulatory bodies
Potential Applications
- Pharmaceutical primary packaging
Pur Q® fused quartz vials, at 99.995% SiO2, have unparalleled purity, owing to the methodical removal and deliberate avoidance of additives and impurities of all types. Control at the single-digit part-per-million level represents a leap forward in expectations for pharmaceutical glass packaging quality. By removing the sources of potential leaching ions and problematic chemical degradation routes, Pur Q® vials greatly reduce risk to formulation stability and efficacy throughout the product lifetime.

What’s Missing Makes It Remarkable
Chemical Durability That’s in a Class of Its Own
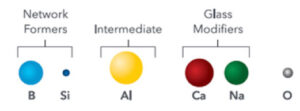
Momentive Pur Q® fused quartz vials are simply 99.995% SiO2. In the bulk, at the surface, at the top, on the bottom, everywhere, every batch, every vial, before heat, after heat, all the time.
For multicomponent glasses such as borosilicate, the local composition, well, varies. Varies by manufacturer, by batch, by thermal history, by geometry, by fill volume, from inside to outside. The multiple elements within the glass can, and frequently, rearrange themselves, creating enriched and depleted areas.
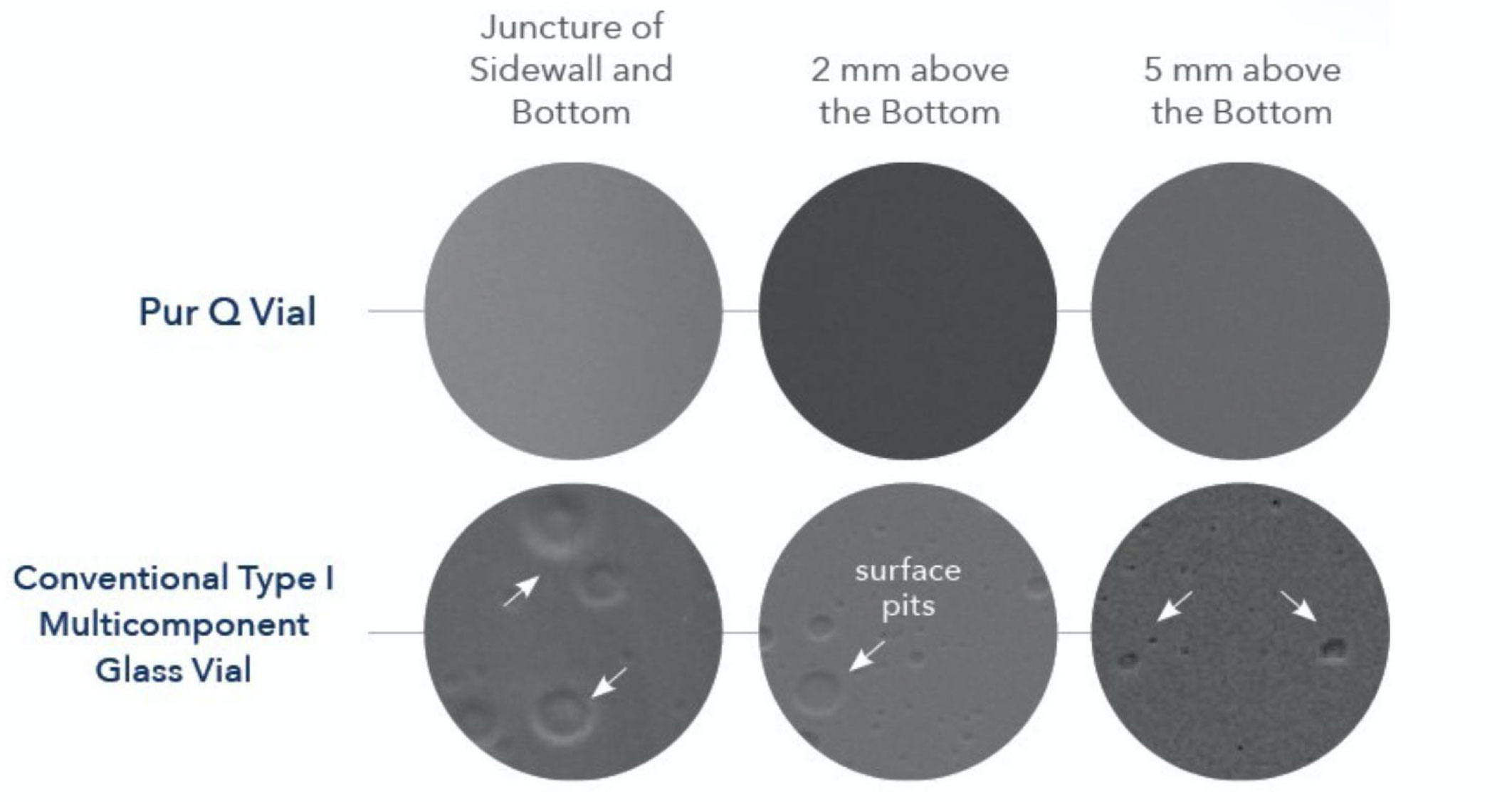
How can I tell if this is happening to my vials? Look for signs of heterogeneous chemical attack like surface pitting, blisters or pits on the inner vial surfaces after exposure to almost any formulation.
See pictures after exposure to water for one hour at 121°F. These are so common that they have unfortunately become expected in Type I vials. Micrographs of the inner vial surfaces following <USP660> hydrolytic stability testing reveal that the Pur Q® vial exhibits none of the features typically observed on conventional Type I vials.
Surface Comparison @ 5000x SEM (after USP 660 Surface Glass Testing)